42 fda requirements food labels
Guidance for Industry: Food Labeling Guide | FDA It is the responsibility for the food industry to remain current with the legal requirements for food labeling. All new regulations are published in the Federal Register (FR) prior to their... A Guide to Federal Food Labeling Requirements for Meat ... A Guide to Federal Food Labeling Requirements for Meat, Poultry, and Egg Products Guideline ID FSIS-GD-2007-0001 Issue Date August 2007 Full Guideline FSIS-GD-2007-0001 This guidance document assists firms in the development of food labels that meet FSIS requirements. This guidance document relates to FSIS labeling regulations in 9 CFR 317 and 381.
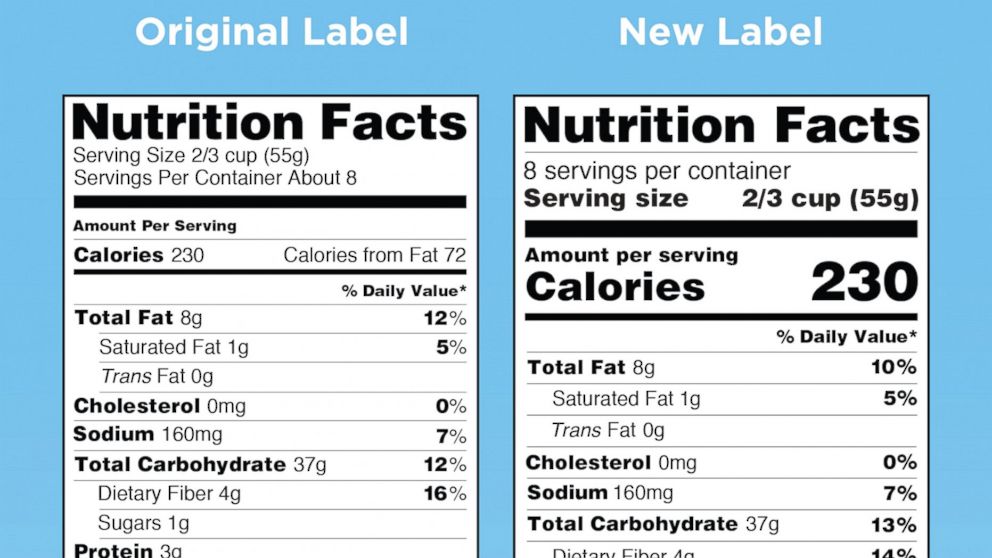
Food Labeling | Food and Nutrition Information Center ... FDA's Food Labeling program develops policy and regulations for dietary supplements, nutrition labeling and food standards, infant formula and medical foods. Also conducts scientific evaluation to support such regulations and related policy development. The New and Improved Nutrition Facts Label-Key Changes
Fda requirements food labels
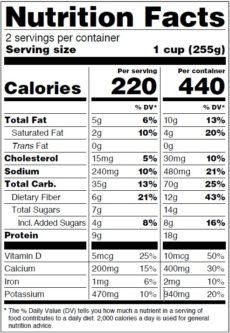
Uniform Compliance Date for Food Labeling Regulations The Food and Drug Administration (FDA or we) is establishing January 1, 2024, as the uniform compliance date for food labeling regulations that are published on or after January 1, 2021, and on or before December 31, 2022. We periodically announce uniform compliance dates for new food labeling requirements to minimize the economic impact of ... FDA Food Product Labeling & Packaging Requirements | ESHA ... Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium) Placement: In general, place the Nutrition Facts Label on the PDP or the Information Panel, near the ingredient statement. Ingredient Statement CFR - Code of Federal Regulations Title 21 - Food and Drug ... Subpart B - Specific Food Labeling Requirements § 101.22 - Foods; labeling of spices, flavorings, colorings and chemical preservatives. § 101.30 - Percentage juice declaration for foods purporting...
Fda requirements food labels. FDA Announces Temporary Food Labeling During COVID-19 Pandemic entitled " temporary policy regarding certain food labeling requirements during the covid-19 public health emergency: minor formulation changes and vending machines ," this guidance is one of... eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label ... Code of Federal Regulations Title 21 - Food and Drug ... (e) A food shall be exempt while held for sale from the requirements of section 403(k) of the act (requiring label statement of any artificial flavoring, artificial coloring, or chemical preservatives) if said food, having been received in bulk containers at a retail establishment, is displayed to the purchaser with either (1) the labeling of ... PDF Food Labeling Guide - FDA Office of Nutrition, Labeling, and Dietary Supplements HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740 (Tel)...
US FDA labeling requirements for food - FDAbasics The above list is just an overview of FDA labeling requirements for food products. Since food labeling is a complex task, as you start working on food labeling, you may face several challenges. Nutrition Facts labeling is one of the most critical parts of food labeling. FDA Food Packaging Guidelines for 2022 | Newprint Nutritional labels must be printed in all black or one colour type on a white or neutral contrasting background and must be readable. There have been changes in the FDA food labeling requirements regarding nutrition facts, and the transition period has ended on January 1, 2021. Health Canada closely regulates the size and appearance of the NFT. Food Labeling 101 - FDA Regulations Guide [2022] | Artwork ... Food Labeling Requirements As Stated By The FDA I. Principal Display Panel 1. Brand Elements 2. Statement of Identity 3. Net Quantity II. Information Panel 1. Ingredient List 2. Instructions to Use 3. Manufacturer Name & Address 4. Country of Origin 5. Product Code III. Nutrient Panel 1. Nutrient Labeling 2. Serving Sizes IV. Claims And Warnings 1. FDA Food Label Compliance - Label Review Fees - fdahelp.us FDA will not review or approve food labels. If the labels are not complying with FDA requirements FDA will consider the product as misbranded and may take regulatory action including detention. LMG's Label review service will help you to confirm your product labels are complying with FDA requirements. we have designed 3 types of label review ...
FDA Labeling Requirements for Food - What You Need to Know Food labeling is required by law for most prepared foods, such as loaves of bread, cereals, canned and frozen foods, desserts, snacks, drinks, etc. Nutrition labeling for raw produce, i.e., fruits and vegetables, and for fish, is voluntary. The FDA refers to these products as "conventional" foods. Menu Labeling Requirements | FDA - U.S. Food and Drug ... The menu labeling requirements apply to restaurants and similar retail food establishments that are part of a chain with 20 or more locations. In addition, they must be doing business under the... PDF A Guide to Federal Food Labeling Requirements for Meat and ... of the states in regulating food labeling is also addressed, along with an explanation of the consistency required between state and federal law. Section II provides an overview of the basic food labeling requirements, including the prior label approval process, establishment FDA Label Search - Food and Drug Administration The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies described in monographs.
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
Overview of FDA Labeling Requirements for Restaurants ... As required by statute, FDA's final rule for nutrition labeling in chain restaurants and similar retail food establishments will provide consumers with clear and consistent nutrition information in...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.100 Food; exemptions from labeling. (a) The following foods are exempt from compliance with the requirements of section 403 (i) (2) of the act (requiring a declaration on the label of the common or usual name of each ingredient ...
Food Ingredients & Packaging | FDA FDA provides regulatory and scientific information about irradiated food and packaging. Irradiation may be used to increase shelf-life and reduce harmful bacteria in meat, poultry, vegetables and...
FDA Food Labeling Requirements | Eric F. Greenberg, P.C. Food, drug and device labeling is heavily regulated, with requirements for both mandatory content as well as voluntary claims made regarding the product. Understanding international and U.S. Food and Drug Administration (FDA) food labeling requirements is essential to ensuring that you remain compliant.
FDA Food Labeling Requirements - Register FDA Firms that file for a Small Business Nutrition Labeling Exemption must meet the following requirements: You must employ less than an average of 100 full-time equivalent employees. You must sell less than 100,000 units of that product in the United States in a 12- month period.
Reliable and Robust Nutrition Food Label Maker Application FDA rules and regulations enforce nutrition food labeling guidelines, so that residents in the U.S. can enhance and manage their health according to personal requirements. On January 1, 2020, the newest food labeling guidelines rolled out for food manufacturers to introduce new nutrition food labels.
Food Labeling Requirements for FDA Compliant Label Design The FDA requires the following business details on food labels: Business name Street address City or town State Zip Code Indicate if the business name is that of the manufacturer, distributor, importer, etc. If the product is exported or manufactured outside of the US, the country of origin must appear conspicuously on the label for food safety.
RL Food Testing Laboratory Issues Warning to Food Manufacturers That FDA Enforcement of New ...
What are the FDA requirements for food- USA Food ... Labeling is one of the important FDA requirements for food products. We can offer labeling review services and our fee for per product labeling review is $ 299. In order to review the label, you should provide us label design in PDF or image format. We offer discounts on multiple labeling reviews.
Code of Federal Regulations Title 21 - Food and Drug ... (a) General requirements. A claim about the calorie or sugar content of a food may only be made on the label or in the labeling of a food if: (1) The claim uses one of the terms defined in this section in accordance with the definition for that term;
Food Product Dating - Food Safety and Inspection Service What Are the Requirements for Dating Infant Formula? Federal regulations require a "Use-By" date on the product label of infant formula under inspection of the U.S. Food and Drug Administration (FDA). Consumption by this date ensures the formula contains not less than the quantity of each nutrient as described on the label.
ESHA Incorporates New FDA Nutrition Facts Labels Into Genesis R&D Food Formulation & Labeling ...
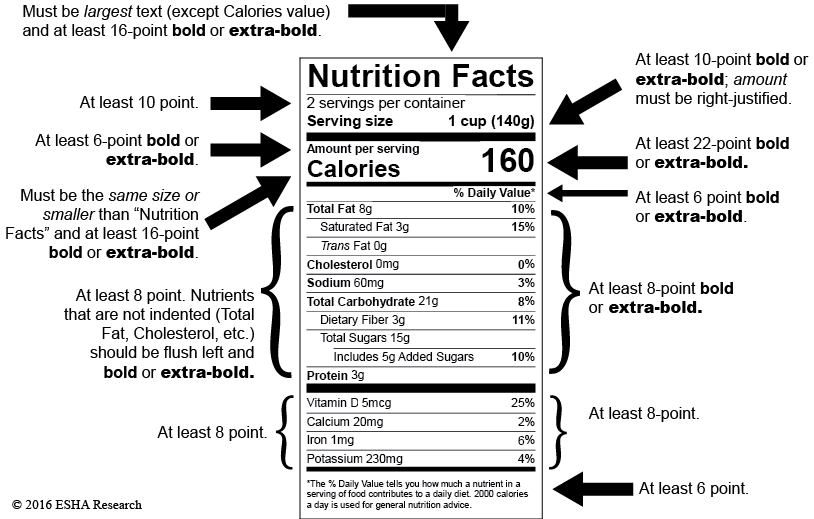
Code of Federal Regulations Title 21 - Food and Drug ... Requirements of conspicuousness and legibility shall include the specifications that: (1) The ratio of height to width (of the letter) shall not exceed a differential of 3 units to 1 unit (no more...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Subpart B - Specific Food Labeling Requirements § 101.22 - Foods; labeling of spices, flavorings, colorings and chemical preservatives. § 101.30 - Percentage juice declaration for foods purporting...
FDA Food Product Labeling & Packaging Requirements | ESHA ... Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium) Placement: In general, place the Nutrition Facts Label on the PDP or the Information Panel, near the ingredient statement. Ingredient Statement
Uniform Compliance Date for Food Labeling Regulations The Food and Drug Administration (FDA or we) is establishing January 1, 2024, as the uniform compliance date for food labeling regulations that are published on or after January 1, 2021, and on or before December 31, 2022. We periodically announce uniform compliance dates for new food labeling requirements to minimize the economic impact of ...
![Food Labeling 101 - FDA Regulations Guide [2021] | Artwork Flow](https://uploads-ssl.webflow.com/5f59aa263c234bb74025de57/5fa4f8a355c6935dd2dde09d_Inner-Images-1.jpg)





Post a Comment for "42 fda requirements food labels"